Learning Outcomes
By the end of this lesson, students will be able to:
i. Define and explain the concept of amorphous solids, recognizing their irregular and disordered atomic arrangements.
ii. Describe the properties of amorphous solids, including their lack of a definite melting point and their glassy appearance.
iii. Define and explain the concept of crystalline solids, understanding their well-defined, repeating atomic arrangements.
iv. Compare and contrast the structural characteristics of amorphous and crystalline solids.
v. Analyze the implications of structural differences on the physical properties of amorphous and crystalline solids.
Introduction
The world of solids is a diverse tapestry of materials, each exhibiting unique properties and characteristics. Among these, amorphous and crystalline solids stand out, their structural distinctions shaping their behavior and influencing their applications.
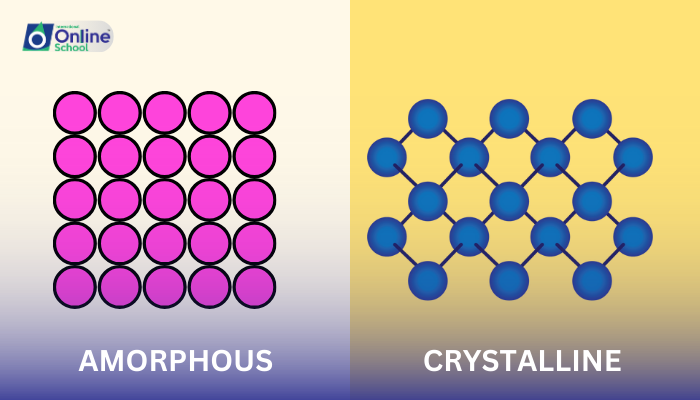
i. Amorphous Solids: A Realm of Disorder
Amorphous solids, also known as non-crystalline solids, are characterized by their irregular and disordered atomic arrangements. Unlike crystalline solids, where atoms or molecules are arranged in a repeating, three-dimensional pattern, amorphous solids lack this order. Their atoms are randomly positioned, resembling a frozen liquid.
Properties of Amorphous Solids
Amorphous solids exhibit several distinct properties:
Lack of a Definite Melting Point: Amorphous solids do not have a sharp melting point. Instead, they soften gradually over a temperature range, transitioning from a rigid solid to a viscous liquid.
Glassy Appearance: Amorphous solids often have a glassy or vitreous appearance, reflecting their disordered atomic structure.
Brittleness: Amorphous solids are typically brittle, shattering easily upon impact due to the absence of a well-defined crystal structure.
ii. Crystalline Solids: A Realm of Order
Crystalline solids, also known as crystal solids, are characterized by their well-defined, repeating atomic arrangements. In these solids, atoms or molecules are arranged in a regular, three-dimensional pattern, forming a crystal lattice.
Properties of Crystalline Solids
Crystalline solids exhibit several distinct properties:
Definite Melting Point: Crystalline solids have a sharp melting point, transitioning from a rigid solid to a liquid at a specific temperature.
Faceted Surfaces: Crystalline solids often have well-defined, flat faces, reflecting their underlying crystal structure.
Mechanical Strength: Crystalline solids are generally more rigid and mechanically strong than amorphous solids due to their ordered atomic arrangement.
Implications of Structural Differences**
The structural differences between amorphous and crystalline solids have profound implications for their physical properties:
Melting Behavior: The disordered structure of amorphous solids leads to their gradual melting over a temperature range, while the ordered arrangement of crystalline solids allows for a sharp transition at a specific temperature.
Mechanical Properties: The lack of a well-defined crystal structure in amorphous solids contributes to their brittleness, while the ordered arrangement of atoms in crystalline solids enhances their rigidity and mechanical strength.
Optical Properties: Amorphous solids can be transparent or opaque, while crystalline solids can exhibit birefringence, the ability to split light into two beams.
Examples of Amorphous and Crystalline Solids**
Amorphous Solids: Glass, plastic, rubber
Crystalline Solids: Table salt, sugar, diamond
Amorphous and crystalline solids represent two distinct classes of solids, each with unique structural characteristics and physical properties. Understanding these distinctions provides valuable insights into the behavior of matter in the solid state and the diverse applications of these materials in various fields, from construction to optics.